By Carol Clark
When an invading bacterium or virus starts rummaging through the contents of a cell nucleus, using proteins like tiny hands to rearrange the host’s DNA strands, it can alter the host’s biological course. The invading proteins use specific binding, firmly grabbing onto particular sequences of DNA, to bend, kink and twist the DNA strands. The invaders also use non-specific binding to grasp any part of a DNA strand, but these seemingly random bonds are weak.
Emory University biophysicists have experimentally demonstrated, for the fist time, how the nonspecific binding of a protein known as the lambda repressor, or C1 protein, bends DNA and helps it close a loop that switches off virulence. The researchers also captured the first measurements of that compaction.
Their results, published in Physical Review E, support the idea that nonspecific binding is not so random after all, and plays a critical role in whether a pathogen remains dormant or turns virulent.
Lysis plaques of lambda phage on E. coli bacteria.
C1 is the repressor protein of the lambda bacteriophage, a virus that infects the bacterial species E. coli, and a common laboratory model for the study of gene transcription.
The virus infects E. coli by injecting its DNA into the host cell. The viral DNA is then incorporated in the bacterium’s chromosome. Shortly afterwards, binding of the C1 protein to specific sequences on the viral DNA induces the formation of a loop. As long as the loop is closed, the virus remains dormant. If the loop opens, however, the machinery of the bacteria gets hi-jacked: The virus switches off the bacteria’s genes and switches on its own, turning virulent.
Finzi runs one of a handful of physics labs using single-molecule techniques to study the mechanics of gene expression. In 2009, her lab proved the formation of the C1 loop. “We then analyzed the kinetics of loop formation and gained evidence that non-specific binding played a role,” Finzi says. “We wanted to build on that work by precisely characterizing that role.”
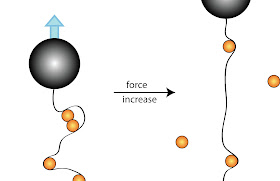
Emory undergraduate student Chandler Fountain led the experimental part of the study. He used magnetic tweezers, which can pull on DNA molecules labeled with miniscule magnetic beads, to stretch DNA in a microscope flow chamber. Gradually, the magnets are moved closer to the DNA, pulling it further, so the length of the DNA extension can be plotted against the applied force.
“You get a curve,” Finzi explains. “It’s not linear, because DNA is a spring. Then you put the same DNA in the presence of C1 protein and see how the curve changes. Now, you need more force to get to the same extension because the protein holds onto the DNA and bends it.”
Specifically-bound proteins are shown as orange ovals on a thicker part of the DNA sequence and non-specifically bound proteins are portrayed as gray ovals on regular DNA. Non-specific, transient loops facilitate the coming together of the specifically-bound proteins that mediate formation of the “switch loop”. Once this loop is formed, non-specifically bound protein further stabilize it by increasing the length of the closure in a zipper-like effect. (Graphic by Monica Fernandez.)
An analysis of the data suggests that, while the specific binding of the C1 protein forms the loop, the non-specific binding acts like a kind of zipper, facilitating the closure of the loop, and keeping it stable until the signal comes to open it.
“The zipper-like effect of the weaker binding sites also allows the genetic switch to be more responsive to the environment, providing small openings that allow it to breathe, in a sense,” Finzi explains. “So the loop is never permanently closed.”
The information about how the C1 genetic switch works may provide insights into the workings of other genetic switches.
“Single-molecule techniques have opened a new era in the mechanics of biological processes,” Finzi says. “I hope this kind of experiment will lead to better understanding of how our own DNA is compacted into chromosomes, and how it unravels locally to become expressed.”
Other authors on the paper include Sachin Goyal, formerly a post-doc in the Finzi lab; Emory cell biologist David Dunlap; and Emory theoretical physicist Fereydoon Family. The research was funded by the National Institutes of Health.
Related:
Undersea cables add twist to DNA research
A physicist's view of life
Image credits: DNA (top) by iStockphoto.com; lamba phage by Madboy via Wikipedia Commons.



No comments:
Post a Comment